It is usually incorrectly thought of rubber as just school rubber.
Due to its flexibility and durability, rubber is a widely used material in various fields. It is an essential item in the fields of car manufacturing, arts and crafts, technology and even in sports.
However, we can also connect this term to latex - a raw ''rubber'' material used in its own or further processed form.
Generally, rubber is an elastomer, a material with great ability to deform and after the deforming power is gone, it returns to its former shape. Latex is more of a fluid, milk-like fluid and it is emulsion of polymers microparticles in aqueous solution.
It is basically present in every plant and it covers many types of molecules - varying from proteins, carbohydrates and oils to alkaloids and resins.
In industry, we can use two types of latex. We have either natural latex or synthetic latex.
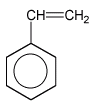
The natural one is by letting the fluid pour out the from gumtree whereas synthetic latex is produced through polymerization of butadiens, usually isopren (2-methylbuta-1,3-dien) or copolymerization of butadien with styren.
isopren
Furthermore, natural rubber is the most important product obtained from latex; more than 12,000 plant species yield latex containing rubber, though in the vast majority of those species the rubber is not suitable for commercial use.This latex is used to make many other products as well, including mattresses, gloves, swim caps, condoms, catheters and balloons.
Latex from the chicle and jelutong trees is used in chewing gum.
In the case of synthetics, rubber is made from latex through interesting process called vulcanization of rubber. It is comprised of adding sulphur to the polymer latex molecules. Sulphur has an interesting characteristics - it can create so-called di-sulphoric bridges (crosslinks) while creating tenuous 3D site and therefore improving some qualities like tensile strength or elasticity (the ability to return from the deformation)
We know for example rubber balls made thanks to this process...
or 30% mass of added sulphur is called ebonite, a very hard and electric-insulating material used in great variety of products varying from bowling balls to saxophone mouthpieces. It replaced expensive ebony wood in the end of 19th century.
Then, we can think of latex like dried latex from the opium poppy, which is opium, the source of many useful opiates and other alkaloids of high value. (morfin - anesthetics, but also heroin - very dangerous drug)
heroine:
When you see someone doing something similar to the last picture, you can be sure he/she is in a very very bad situation :-(
Finally, we can think of latex of course in clothing! Worn on the body (or applied directly by painting) it tends to be skin-tight, producing a "second skin" effect. :-)
For now, you should have a little bit better idea, when we say ''gum'' or rubber''
But be aware, especially in the case of clothing - do not put a latex head or whatever over your mouth and nose, ''rubber'' materials can be of different quality, but be sure with one thing - one cannot breath through it :-)
Sources:
http://www.solarnavigator.net/rubber.htm
http://en.wikipedia.org/wiki/Latex
http://cs.wikipedia.org/wiki/Kau%C4%8Duk
http://xantina.hyperlink.cz/organika/uhlovodiky/areny.html
http://www.jergym.hiedu.cz/~canovm/polymery/polymera/pbdstk.htm
http://en.wikipedia.org/wiki/Vulcanization
http://www.viewzone.com/chewinggum.html
http://singlets.co.cc/late-19th-centuary-clothing.html
http://bowlingtipsandtechniques.com/your-options-on-finding-a-great-bowling-ball/